Results for 'anticancer therapy'

Leadership Lab: 4 Ways Biopharma Leaders Can Prepare for Media Interviews
Aug 20th • 6 mins read

Presentation Nails and Fails: 7 Tips to Ace Your Next MSL Presentation
Oct 12th • 1 min read




Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006-2020
Apr 20th • 7 mins read

Comparative study on anticancer drug access times between FDA, EMA and the French temporary authorisation for use program over 13 years
Apr 7th • 12 mins read

Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
Feb 23rd • 8 mins read

The First 2 Years of Biosimilar Epoetin for Cancer and Chemotherapy-Induced Anemia in the U.S.: A Review from the Southern Network on Adverse Reactions
Mar 12th • 7 mins read
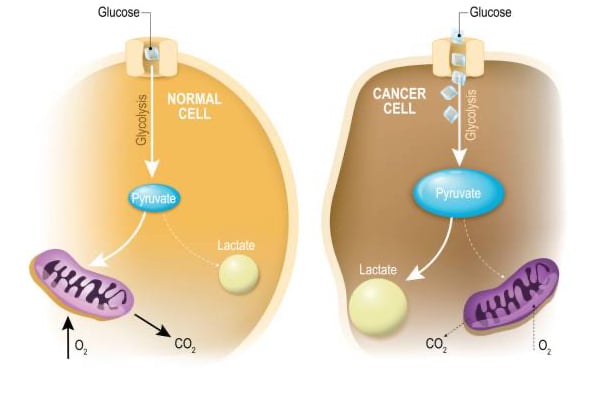
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read
Confounding factors in exposure–response analyses and mitigation strategies for monoclonal antibodies in oncology
Nov 20th • 12 mins read

Seven decades of chemotherapy clinical trials: a pan-cancer social network analysis
Oct 16th • 12 mins read

Liquid biopsy in oncology: a consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology
Sep 26th • 17 mins read

Leveraging existing data to contextualize phase II clinical trial findings in oncology
Sep 21st • 3 mins read
A Field Test of Major Value Frameworks in Chemotherapy of Nasopharyngeal Carcinoma-To Know, Then to Measure
Aug 12th • 10 mins read

Application of the ESMO-Magnitude of Clinical Benefit Scale (V.1.1) to the field of early breast cancer therapies
Sep 6th • 20 mins read
Lessons learnt from scoring adjuvant colon cancer trials and meta-analyses using the ESMO-Magnitude of Clinical Benefit Scale V.1.1
Sep 6th • 17 mins read
Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration
Aug 31st • 16 mins read

Past, Current, and Future Cancer Clinical Research Collaborations: The Case of the European Organisation for Research and Treatment of Cancer
Aug 16th • 8 mins read

Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration
Jul 22nd • 12 mins read
